
Enumeration of Escherichia coli and coliform bacteria in 18 hours
COLIKAT® RAPID is a fast test to simultaneously detect both total coliforms and Escherichia coli in water samples providing the test’s results within 18 hours. COLIKAT® RAPID is suited for the entire water matrix.
The water samples to be tested are incubated overnight so that they are ready to read early the next day. Therefore COLIKAT® RAPID delivers significant workflow benefits and speed in the event of a contamination issue. The test’s high sensitivity enables early coliform detection in water distribution systems, assisting to help to reduce biofilm buildup and sporadic downstream contamination events. As compared to the alternative conventional method using Chromogenic Coliform Agar (CCA; ISO 9308-1) for detection, COLIKAT® RAPID significantly reduces false alarms created by inaccurate false positive results which give rise to uncertainty and unnecessary expense.
- Simultaneous detection and quantification of Escherichia coli and total coliforms for the entire water matrix.
- Sample evaluation after 18 hours.
- Optimized workflow in your laboratory: The sample evaluation takes place after 18 hours before the reception of next days’ samples.
- Enumeration of Escherichia coli and coliform bacteria in water samples acc. to EN ISO 9308-2.
Tap Water
Bottled Water
Sea Water
Bathing Water
Waste Water
Surface Water
Summary of EN ISO 17994 Study results:
“The study clearly proved that the XEBIOS‘s COLIKAT® RAPID method is equivalent to the IDEXX‘s COLILERT-18/QUANTI-Tray® method for the enumeration of coliform bacteria.
For the target organism E. coli the study clearly proved that XEBIOS‘s COLIKAT® RAPID has a significantly higher recovery compared to IDEXX‘s reference method COLILERT- 18®/QUANTI-Tray®.”
IWW Rheinisch-Westfälisches Institut für Wasser Beratungs- und Entwicklungsgesellschaft mbH, January 2021 [Final report of a comparison study for the evaluation of the equivalence of XEBIOS DIAGNOSTICS COLIKAT RAPID® and IDEXX LABORATORIES COLILERT-18®, page 18], Download here.
- IWW Rheinisch-Westfälisches Institut für Wasser Beratungs- und Entwicklungsgesellschaft mbH as independent and internationally recognized experts have carried out a comparison study on behalf of Xebios to evaluate the equivalence of Colikat Rapid® and Idexx Laboratories Inc. Colilert-18® as reference method.
- The study has been carried out in accordance with the requirements of EN ISO 17994:2014: Water Quality – Requirements for the comparison of the relative recovery of microorganisms by two quantitative methods.
- 522 sample-pairs were used for the comparison of the test systems’ performance for the enumeration of the key target organism Escherichia coli, 470 sample-pairs for coliform bacteria were included into the study – providing a profound database to guarantee statistically sound statements within EN ISO 17994’s test design.
- Colikat Rapid® has a significantly higher recovery for E. coli compared to Idexx Laboratories Inc. Colilert-18®
- both methods are equivalent regarding the enumeration of coliform bacteria.
Products available now

COLIKAT RAPID
COLIKAT RAPID

- Suitable for presence-/absence testing and simultaneous identification and quantification of Escherichia coli and coliform bacteria using the COLIKAT Blister
- Easy to use without extensive training of laboratory staff in the laboratory or in the field
- Clear identification of target organisms avoids results’ bias resulting from subjective interpretation
- No confirmation testing required
- Detection of 1 CFU/100ml water sample
- 18 hours test period perfectly fits into laboratory workflow and helps to avoid night and weekend work
- 24 months shelf life at room temperature
- Low percentage of false positive results increases security and avoids unnecessary cost
- Quality tested according to EN ISO 11133 and EN ISO 9308-2 in our EN ISO 17025 accredited laboratory
- Test is compliant with §15 Sec. 1a Nr. 1 of TrinkWV and European Union Directive 2015/1787 EC
COLIKAT
Enumeration Tray
COLIKAT Enumeration Tray
- For the simultaneous identification and quantification of Escherichia coli and coliform bacteria
- 3-side pre-sealing insures easy filling of the COLIKAT Enumeration Tray
- Easy and fast sealing to separate the cavities with the COLIKAT Seal Applicator
Download Enumeration Tray 51 (PDF)
Download Enumeration Tray 97 (PDF)
XDG Multiple Well MPN Tray Sealer
XDG Multiple Well MPN Tray Sealer
- Compact sealer to seal the COLIKAT Enumeration Tray
- Safe separation of the cavities for valid quantification
COLIKAT
Sample Vessels
COLIKAT Sample Vessels
- 125ml and 300ml vessels
- Available with and without sodium thiosulfate
- Available with and without anti-foaming agent
COLIKAT
Compare Kit
COLIKAT Compare Kit
- To assist distinguishing positive from negative results by comparing the color of the Compare Kit with the color of the sample
QC
Strains
QC Strains
- Escherichia coli (WDCM 00090)
- Escherichia coli (WDCM 00013)
- Klebsiella pneumoniae (WDCM 00206)
- Pseudomonas aeruginosa (WDCM 00024)
| Code | Product name | Details |
|---|---|---|
| 95-1901 | COLIKAT RAPID® (18h) | 100 tests for 100ml samples in stand-up pouch |
| 95-1902 | COLIKAT® Enumeration Tray 51 | 100 Enumeration Trays in bag |
| 95-1904 | COLIKAT® Enumeration Tray 97 | 80 Enumeration Trays in bag |
| 95-1909 | COLIKAT® Antifoam Agent | 20ml for 200 tests |
| 95-1910 | Sample vessels 120ml w. sodium thiosulfate, shrink band | 100 vessels, sterile, 100ml mark |
| 95-1911 | Sample vessels 120ml, shrink band | 100 vessels, sterile, 100ml mark |
| 95-1912 | Sample vessels 120ml, tear-of-label | 100 vessels, sterile, 100ml mark |
| 95-1913 | Sample vessels 120ml w. sodium thiosulfate, tear-of-label | 100 vessels, sterile, 100ml mark |
| 95-1915 | COLIKAT® Comperator Enumeration Tray 51 | 1 Comperator to compare samples |
| 95-1916 | COLIKAT® Comperator Enumeration Tray 97 | 1 Comperator to compare samples |
| 95-1917 | COLIKAT Comperator Vessel | 1 Sample Vessel 120ml |
| 95-1924 | COLIKAT® 24 | 100 tests for 100ml samples |
| 95-1930 | COLIKAT® RTU | 25 tests for 100ml, reagent in vessel |
| 95-1931 | COLIKAT® RTU | 25 tests for 100ml, reagent w/ thiosulfate in vessel |
| 95-1934 | COLIKAT RAPID® RTU KIT | 100 tests for 100ml (COLIKAT RAPID, 51 tray, vessel) |
| 95-1935 | COLIKAT RAPID® RTU KIT | 100 tests for 100ml (COLIKAT RAPID, 97 tray, vessel) |
| 95-1940 | ENTEROKAT (coming soon) | 25 tests for 100ml samples |
| 95-1941 | ENTEROKAT (coming soon) | 100 tests for 100ml samples |
| 95-1950 | XDG Multiple Well MPN Tray Sealer | 1 thermal sealing device to seal MPN Enumeration Tra |
Products benefits:
- Significantly higher recovery for the key target organism Escherichia coli
- 1:1 equivalent results regarding coliform bacteria
- The COLIKAT RAPID® reagent dissolves significantly faster in the sample
- No antifoam is needed for the majority of water samples
- Saves more then 80% volume of waste and storage area for the COLIKAT RAPID® reagent
- Manufactured and distributed by your reliable service partners Xebios Diagnostics GmbH and our local established and experienced distribution partners
Test Principle
COLIKAT RAPID® uses certain specific characteristics of both Escherichia coli and coliform bacteria for identification of the species. The test contains MUG and ONPG, two common nutritional indicators. Coliform bacteria use their enzyme β-Galactosidase to metabolize ONPG turning the sample yellow. Escherichia coli – other then most coliform bacteria – additionally has the enzyme β-Glucuronidase that enables it to hydrolyze MUG so that the sample additionally shows fluorescence under UV-light.
Most of non-coliform bacteria possess neither the β-Galactosidase nor the β-Glucuronidase. Therefore, they cannot transform MUG and ONPG and the test will not detect them. Yet, some non-coliform bacteria contain the enzymes. COLIKAT RAPID® therefore uses selective supplements that inhibit the growth of those bacteria. The combination of the test’s enzymes and selective supplements prevents some common problems of conventional culture methods such as Chromogen Coliform Agar CCA (EN ISO 9308-1):
- False positive results are prevented (for example for Aeromonas spec.)
- The growth of non-target organism is prohibited. This way non-target organisms cannot affect (or even inhibit) the growth of Escherichia coli and coliform bacteria
Application – Instructions for use
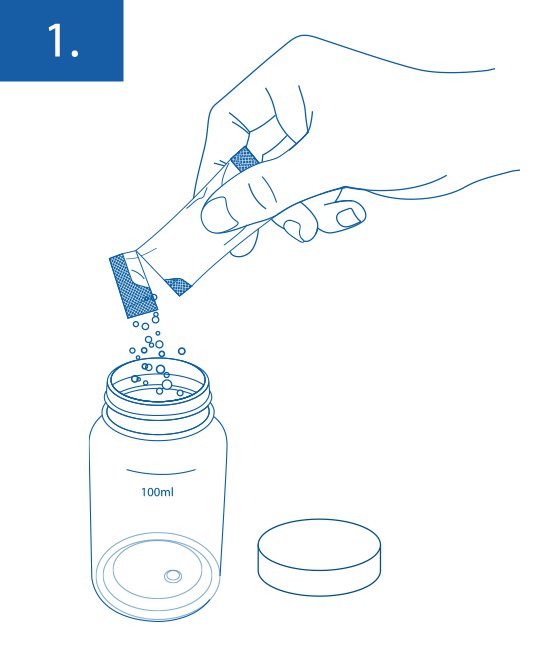
Presence/ Absence: Add 1 bag of COLIKAT RAPID® to 100 ml of water sample. Close the sample vessle and shake until the reagent dissolves.

For presence/absence testing incubate the closed vessel for 18-22 hours at 36°C ± 2°C and read-off the result. The vessels should have room temperature before incubation. If turned yellow coliforms are present. If it shows fluorescence unter UV-light Escherichia coli is present.
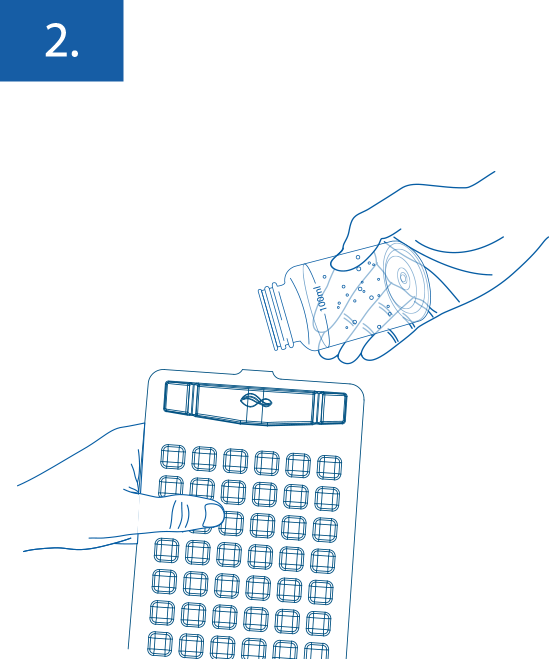
Quantification: For quantitative testing pour the 100ml sample into the COLIKAT Enumeration Tray.
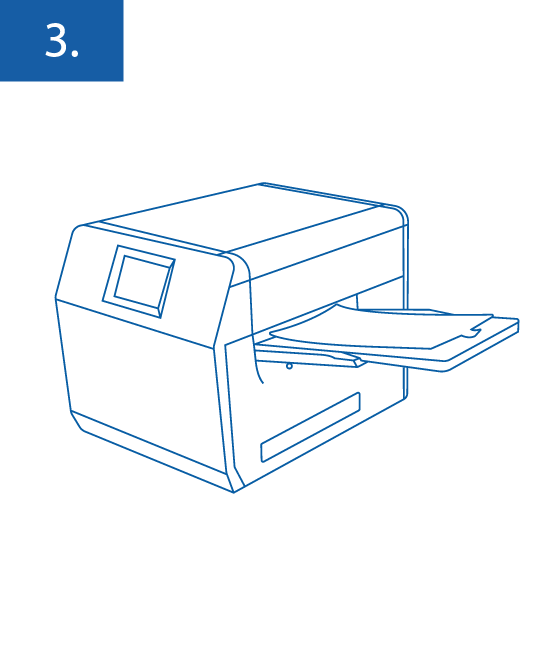
Seal the Enumeration Tray with the XDG Multiple Well MPN Tray Sealer or another enumeration tray sealing device.

Incubate the sample 18-22 hours at 36°C ± 2°C. Count the yellow wells of COLIKAT Enumeration Tray (= coliforms). Count the yellow wells of the COLIKAT Enumeration Tray that show fluorescence under an UV light (365nm) in a dark environment (= Escherichia coli).
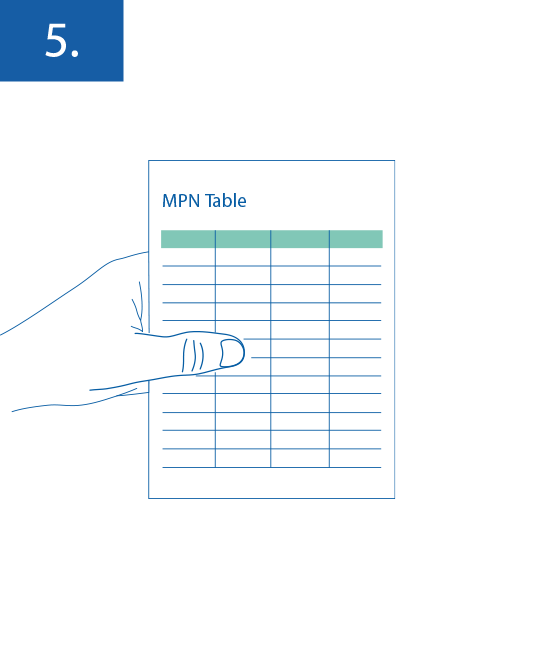
Transform the count results to the number of CFU the sample by using the MPN table in the appendix to EN ISO 9308-2.
Regulatory Environment and Compliance
The requirements for the quality of water intended for human consumption within the European Union are bindingly set by EU regulation:
- DIRECTIVE 98/83/EC – quality of water intended for human consumption
- DIRECTIVE 2015/1787 EC amending Annexes II and III to Council Directive 98/83/EC on the quality of water intended for human consumption
A number of ISO standards have been established for analyzing microbiological parameters. EN ISO 9308-1 and EN ISO 9308-2 (for the enumeration of Escherichia coli and coliform bacteria) provide all necessary specifications for performing the analysis of Escherichia coli and coliform bacteria. The actual standards to be applied, reflecting the latest scientific and technical progress, are reflected by the amendments to Annex III of Directive 98/83/EC. The microbiological parameters and methods of analysis are specified in Annex III to Directive 98/83/EC that has been amended as follows by Directive 2015/1787 EC:
“The methods for microbiological parameters are:
(a) Escherichia coli (E. coli) and coliform bacteria
(EN ISO 9308-1 or EN ISO 9308-2)
(b) Enterococci (EN ISO 7899-2)
(c) Pseudomonas aeruginosa (EN ISO 16266)
(d) enumeration of culturable microorganisms — colony count 22 °C
(EN ISO 6222)
(e) enumeration of culturable microorganisms — colony count 36 °C
(EN ISO 6222)
(f) Clostridium perfringens including spores (EN ISO 14189)’;
The directive requires the member states to implement ISO 9308-2 into national legislation: According to Directive 2015/1787 EC, “Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with this Directive by 27 October 2017 at the latest. They shall forthwith communicate to the Commission the text of those provisions.” As a result of the European legislation, all member states will have to accept EN ISO 9308-2 as method for the analysis of water samples of water intended for human consumption to ensure compliance with EU legislation. Germany has already implemented the new EU regulation into national law:
In Germany, the relevant law to comply with Directive 2015/1787 EC is the “Trinkwasserverordnung” (TrinkWV): “Diese Verordnung dient der Umsetzung der Richtlinie (EU) 2015/1787 der Kommission vom 06. Oktober 2015 zur Änderung der Anhänge II und III der Richtlinie 98/83/EG des Rates über die Qualität von Wasser für den menschlichen Gebrauch.“
The TrinkWV has been adapted to comply with the new EU regulation (with delay) on 03. January 2018. The new TrinkWV has been published in the “Bundesgesetzblatt” and therefore has become effective on 08. January 2018. According to § 15 (1a) Nr. 1 the following methods are to be used for the analysis of drinking water: “Coliforme Bakterien und Escherichia coli (E. coli): DIN EN ISO 9308-1:2017-09 oder DIN EN ISO 9308-2:2014-06.”
DIN EN ISO 9308-2:2014-06 states
(a) the typical composition of the medium to be used.
(b) that the circumstance, that the method has been named as branded by its inventor, does clearly not state exclusivity to produce and use the formulation described by EN ISO 9308-2.
Quality
The manufacturing, storage and quality control of COLIKAT RAPID® is carried out by Xebios Diagnostics GmbH in compliance with EN ISO 11133 and considering Good Manufacturing Practice (GMP). The quality management system is ISO 9001 certified. The quality testing of COLIKAT RAPID® takes place in our EN ISO 17025 accredited laboratory complying with all requirements of EN ISO 11133 and EN ISO 9308-2. It is our goal to meet our customers’ high quality requirements at all time.
Our quality management system includes broad testing procedures for sterility, stability and shelf live, the microbiological performance and physical condition of our products. We trust only established partners which have proven to delivery according to our high quality standards for years for the sourcing of our raw materials and technical equipment.
Distribution Partners
Contact and Support
Please contact us at any time for all your questions about our products and services as well as for an individual quotation.
You can reach our team by phone, e-mail or by using our contact form below.
Xebios Diagnostics Group GmbH
Telleringstraße 49
40597 Düsseldorf
T : +49 211 78 88 07 – 00
E : sales@xebios.de


